New Class of Antibiotics: The Promise of Turnercyclamycins
In the ongoing battle against antibiotic resistance, scientists are turning to the most unlikely of places for solutions: the depths of the ocean and the guts of wood-eating shipworms. Meet turnercyclamycins, a new class of antibiotics derived from the symbiotic bacteria of shipworms, which could revolutionize our fight against drug-resistant infections. These lipopeptide antibiotics are not only effective against some of the most challenging Gram-negative pathogens but also offer hope in combating colistin-resistant strains, a growing threat in modern medicine.
But what makes turnercyclamycins so special? And how did we discover them? The story begins with a bizarre, nightmarish creature: the giant shipworm.
The Giant Shipworm: A Living Laboratory
Imagine a creature that looks like a mix of wet lips, diseased tonsils, and a glistening blue tube stretching up to four feet long. This is the giant shipworm, a marine mollusk that lives in a tusklike tube made of calcium carbonate. Unlike its smaller relatives, which feed on wood, the giant shipworm has evolved a unique way of surviving: it relies on symbiotic bacteria in its gills to convert toxic hydrogen sulfide from decaying wood into energy.
This strange relationship between the shipworm and its bacteria has fascinated scientists for decades. In 2017, researchers led by Dr. Daniel Distel of Northeastern University finally uncovered the secrets of this elusive creature. By studying its genome and the bacteria living within its cells, they discovered that the shipworm’s bacteria produce a suite of bioactive compounds, including turnercyclamycins, which play a critical role in defending both the bacteria and the shipworm from infections.
Want to know best exercises for weight loss
Turnercyclamycins: A New Weapon Against Superbugs
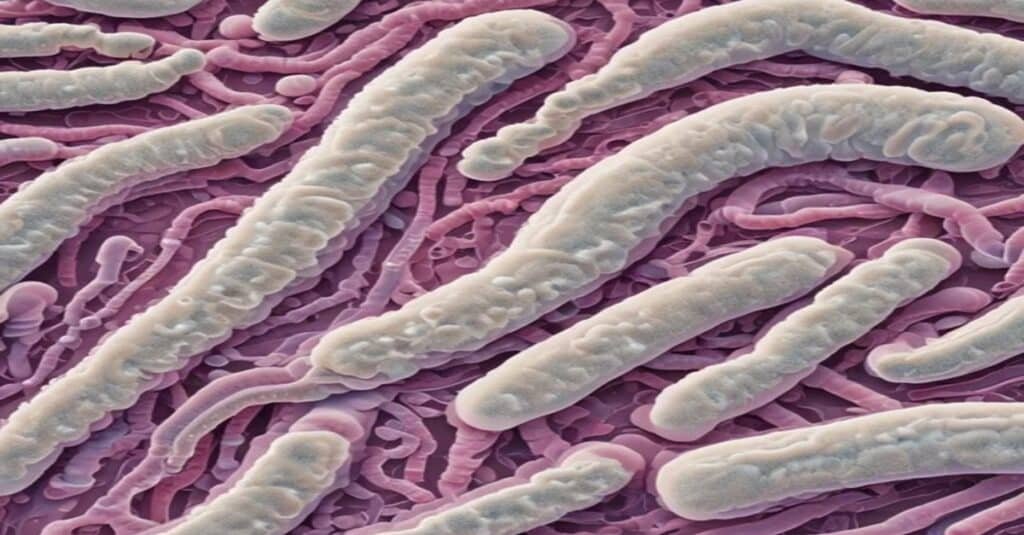
Turnercyclamycins are lipopeptide antibiotics encoded in the genomes of Teredinibacter turnerae, the symbiotic bacteria found in shipworms. These compounds have shown remarkable bactericidal activity against Gram-negative pathogens, including Acinetobacter baumannii, a notorious superbug responsible for hospital-acquired infections. What makes turnercyclamycins particularly exciting is their ability to kill colistin-resistant strains, which are increasingly common and difficult to treat.
How Do Turnercyclamycins Work?
- Targeting the Outer Membrane: Turnercyclamycins disrupt the outer membrane of Gram-negative bacteria, similar to colistin. However, they operate through a slightly different molecular mechanism, allowing them to bypass colistin resistance.
- Broad-Spectrum Activity: These antibiotics are effective against a range of pathogens, making them a versatile tool in the fight against multidrug-resistant infections.
- Evolutionary Advantage: By studying the symbiotic relationship between shipworms and their bacteria, scientists have tapped into millions of years of evolutionary innovation to discover compounds that address unmet medical needs.
The Science Behind the Discovery
The discovery of turnercyclamycins is a testament to the power of exploring unconventional environments. Shipworms, often considered pests for their ability to destroy wooden ships and piers, have turned out to be a treasure trove of bioactive compounds. Researchers analyzed the genomes of Teredinibacter turnerae and identified several biosynthetic gene clusters responsible for producing turnercyclamycins. These clusters are conserved across shipworm populations worldwide, suggesting that the antibiotics play a crucial role in their survival.
In addition to turnercyclamycins, scientists have uncovered other fascinating compounds from shipworm symbionts, such as teredinibactins, which regulate metal ions like iron, copper, and molybdenum. These discoveries highlight the intricate chemical interplay between shipworms and their bacterial partners, offering insights into both marine biology and human medicine.
Why This Matters
Antibiotic resistance is one of the greatest public health challenges of our time. According to the World Health Organization, drug-resistant infections could claim 10 million lives annually by 2050 if no action is taken. Turnercyclamycins represent a beacon of hope in this dire landscape. By targeting the outer membrane of Gram-negative bacteria—a structure that has proven difficult to penetrate with existing antibiotics—they offer a new avenue for treatment.
Moreover, the discovery of turnercyclamycins underscores the importance of bioprospecting in unique environments. From the depths of the ocean to the guts of shipworms, nature has already developed solutions to many of the challenges we face. By studying these natural systems, we can harness the fruits of evolution to address urgent health needs.
Read for losing Weight Loss
What’s Next?
While turnercyclamycins are still in the preclinical stage, their potential is undeniable. Researchers are now working to optimize these compounds for clinical use, ensuring they are both effective and safe for humans. If successful, turnercyclamycins could join the arsenal of next-generation antibiotics, providing a much-needed weapon against superbugs.
In the meantime, the story of the giant shipworm and its symbiotic bacteria serves as a reminder of the wonders that await discovery in the natural world. As Dr. Distel aptly put it, “Whenever you find something so weird and so unusual, there’s often going to be unexpected discoveries that come from it.”
Conclusion
Turnercyclamycins are more than just a new class of antibiotics—they are a testament to the power of curiosity, collaboration, and the untapped potential of the natural world. By studying the bizarre and the beautiful, we can uncover solutions to some of humanity’s most pressing problems. The giant shipworm, once a living nightmare, may yet become a beacon of hope in the fight against antibiotic resistance.
So, the next time you hear about a strange creature from the depths of the ocean, remember: it might just hold the key to saving millions of lives.
To read more innovation articles click here.